FIRST-IN-CLASS PIPELINE
Pipeline
Starting with a therapeutic-area agnostic approach, we have pursued proprietary lead molecules with potential in oncology and autoimmune disease. We are unafraid of tackling the unknown and passionate about creating a first-in-class pipeline that will deliver for patients with significant unmet needs.
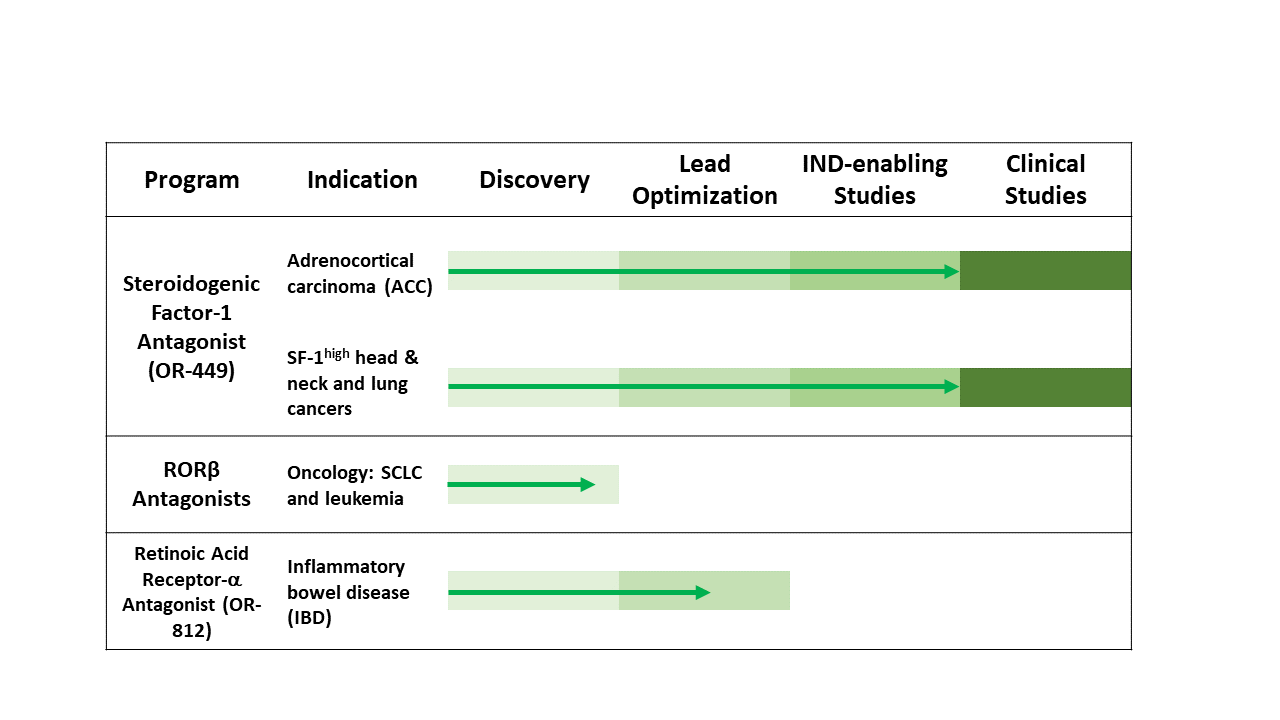
Partnerships are a key element of our continued success in advancing our programs. Orphagen successfully partnered its first program for ROR-gamma antagonists with a mid-sized pharmaceutical company ahead of all competitors in the field. Funding from its partnerships and other non-dilutive sources, including federal grants, has allowed Orphagen to advance its proprietary first-in-class drug discovery programs, including OR-449 for cancers such as adrenocortical cancer.
- OR-449: Orphagen’s oncology lead candidate OR-449 is a potentially major step forward in treatment for adrenocortical carcinoma (ACC), an aggressive cancer of the adrenal gland. A silent killer with no effective treatment and only modest improvements in medical therapy in over 50 years, ACC leaves metastatic patients with limited options and little hope for long-term survival.
- Promising expansion indications for OR-449 include subsets of head & neck and lung squamous cancers (about 10% and 3% respectively), where SF-1 is ectopically elevated along with a distinct genomic signature strongly indicating that tumor survival depends on SF-1.
- Now at an exciting turning point for Orphagen, we are poised for IND filing with OR-449 and the initiation of Phase 1 clinical trials in adult ACC and other SF-1high tumors.
- The U.S. Food and Drug Administration (FDA) has granted OR-449 a Rare Pediatric Disease Designation (RPDD), meant to incentivize rapid development of OR-449 for the treatment of the pediatric form of adrenocortical ACC where SF-1 is somatically amplified.
- Beyond OR-449, Orphagen has built a diverse pipeline with additional first-in-class drug discovery programs that modulate the transcriptional activity of other unexploited orphan nuclear receptors.
- RORβ antagonists. Promising therapeutic opportunities with preclinical pharmacology and/or genomic support include SCLC and certain leukemias. Our unique small molecule tools equip us to engage in collaborations to understand the pharmacological and therapeutic potential of this novel drug target.
- OR-812 is a retinoic acid receptor alpha antagonist currently in lead exploration for inflammatory bowel disease (IBD) and other diseases of intestinal inflammation. Current IBD therapy is dominated by injectable drugs. The small molecule OR-812 will offer a novel form of oral therapy with a differentiated mechanism of action.
