FIRST-IN-CLASS PIPELINE

Pipeline
Starting with a therapeutic-area agnostic approach, we have pursued proprietary lead molecules with potential in oncology and autoimmune disease. We are unafraid of tackling the unknown and passionate about creating a first-in-class pipeline that will deliver for patients with significant unmet needs. Our goal is to develop novel oral drugs for chronic disease.
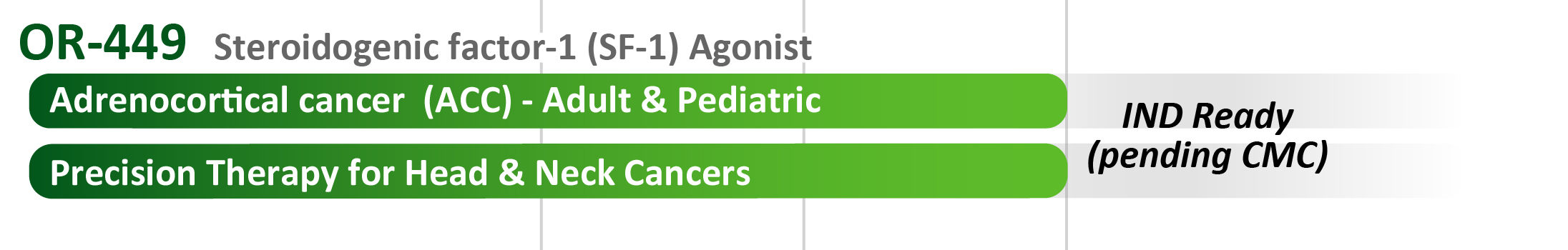
- OR-449: Our most advanced program, soon to enter the clinic in oncology, is a first-in-class orally-bioavailable antagonist for the orphan nuclear receptor steroidogenic factor-1 (SF-1, NR5A1). A major indication is the rare and largely untreatable cancer of the adrenal gland, also known as adrenocortical cancer (ACC), where SF-1 is recognized as a promising therapeutic target [1]. There are potential expansion indications for OR-449 (as a precision therapy) in head & neck, lung squamous, and other cancers where SF-1 is found to be highly elevated in discrete subsets of each tumor type.
- ACC is an aggressive cancer that includes both pediatric and adult populations. Metastatic patients have very limited options for effective treatment. SF-1, the target of OR-449, is a widely-used marker for histological confirmation of ACC.
- The U.S. Food and Drug Administration (FDA) has granted OR-449 a Rare Pediatric Disease Designation (RPDD), meant to incentivize rapid development of OR-449 for the treatment of the pediatric form of adrenocortical ACC.
- The Texas State cancer agency, CPRIT, has awarded Orphagen a $10.2 M grant to support IND filing and a Phase 1 clinical trial for OR-449 in adult ACC. The grant extends for three years and depends on matching funds to be raised by Orphagen.
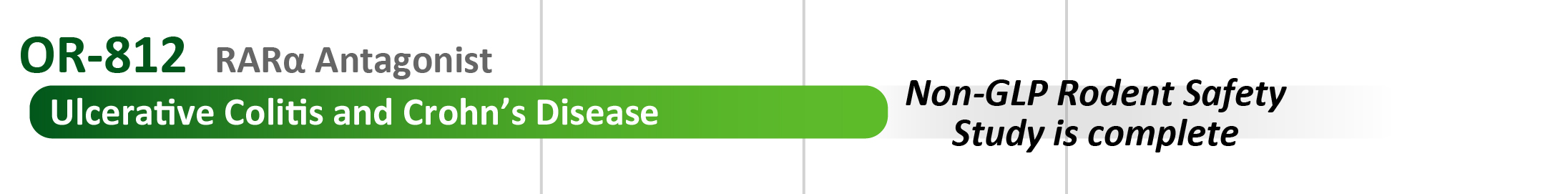
- OR-812: An antagonist of the retinoic acid receptor alpha (RARα) and a development candidate for inflammatory bowel disease (IBD) and other diseases of intestinal inflammation. Current IBD therapy blocks just a handful of potential therapeutic pathways. The small molecule OR-812 will offer a novel form of oral therapy with a differentiated mechanism of action.
- A $1.7 M award in September 2024 from the National Institutes of Health to support further development of OR-812 for IBD.
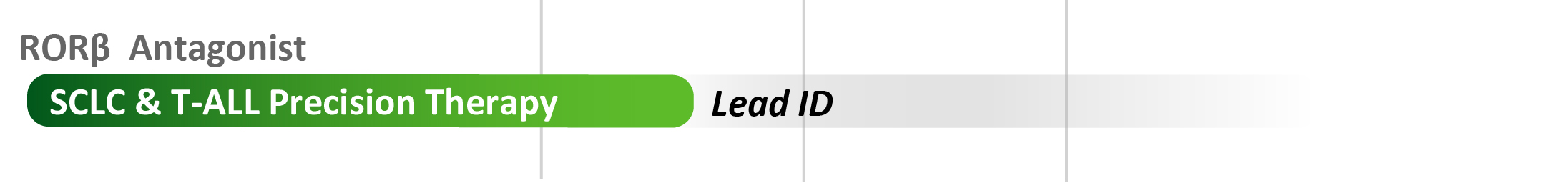
- RORβ antagonists: Promising therapeutic opportunities with preclinical pharmacology and/or genomic support include SCLC and certain leukemias. Our unique small molecule tools equip us to engage in collaborations to understand the pharmacological and therapeutic potential of this novel drug target.
[1] Lerario et al (2022). Endocr Rev 43(6): 1051-1073.